NaDCC is not a hypochlorite – it has unique properties
- It is a fast acting, powerful biocide
- NaDCC dissolves in water to release hypochlorous acid (HOCl) and monosodiumcyanurate (a non-toxic biodegradable compound)
- HOCl is the biocidal agent responsible for killing microorganisms
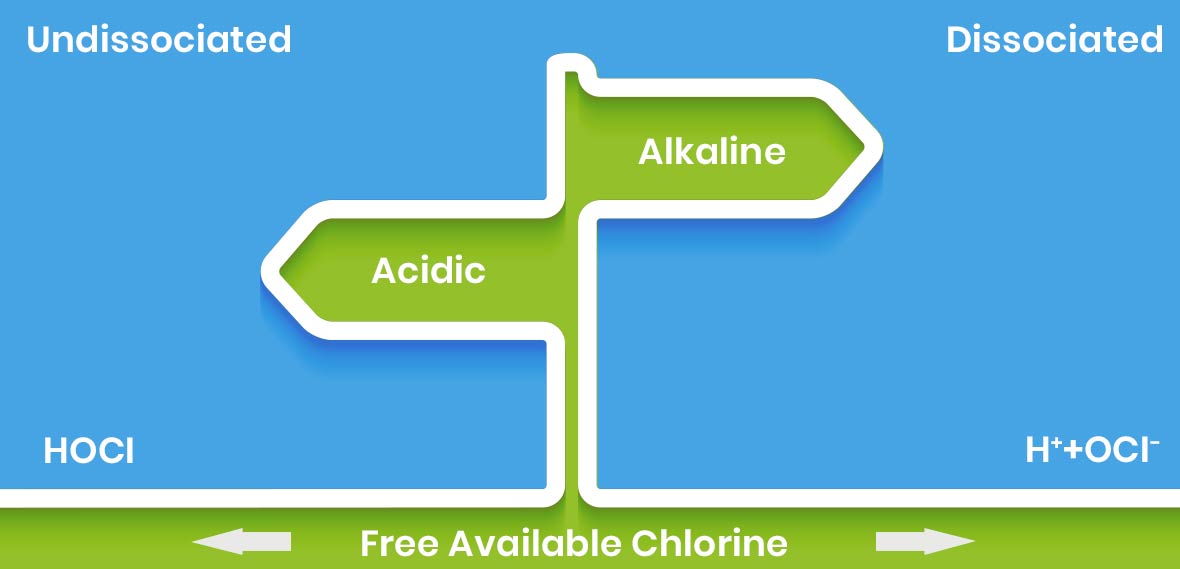
- HOCl dissociates in alkaline conditions into the hydrogen ion (H+) and hypochlorite ion (OCl-)
NaDCC has unique properties that make it a superior chlorine donor and distinctly more effective than hypochlorites:
- Close to pH neutral
- Extended residual killing power due to chemical equilibrium effect
Giving:
- Superior biocidal performance
- Protection from organic matter
Undissociated HOCl is 100 times more powerful than the dissociated components
Why Is HOCl 100 Times More Powerful?
- HOCl has a similar chemical structure to water (HOH)
- It is similar in size and it is electrically neutral
- These factors enable it to penetrate the cell wall in a similar way to water
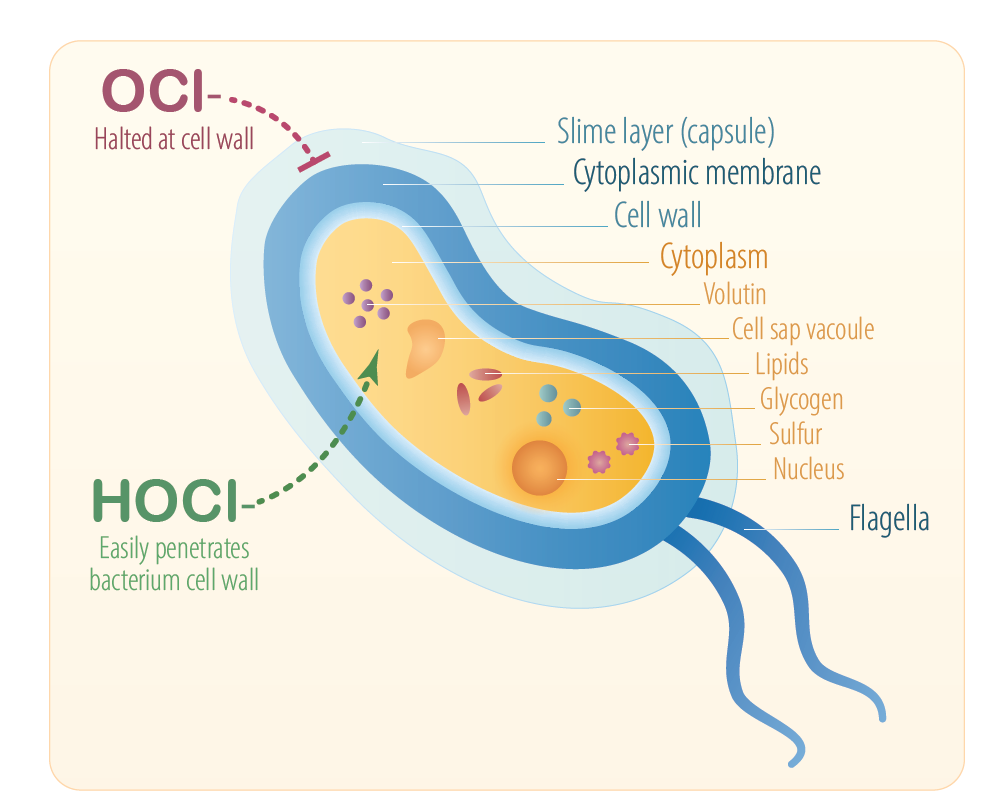
OCl– is electrically charged, which makes it difficult to penetrate the cell wall
It is believed that, after diffusing through the cell wall, HOCl destroys the proteins and enzyme systems essential to life, by an oxidation process.
Organic Contamination
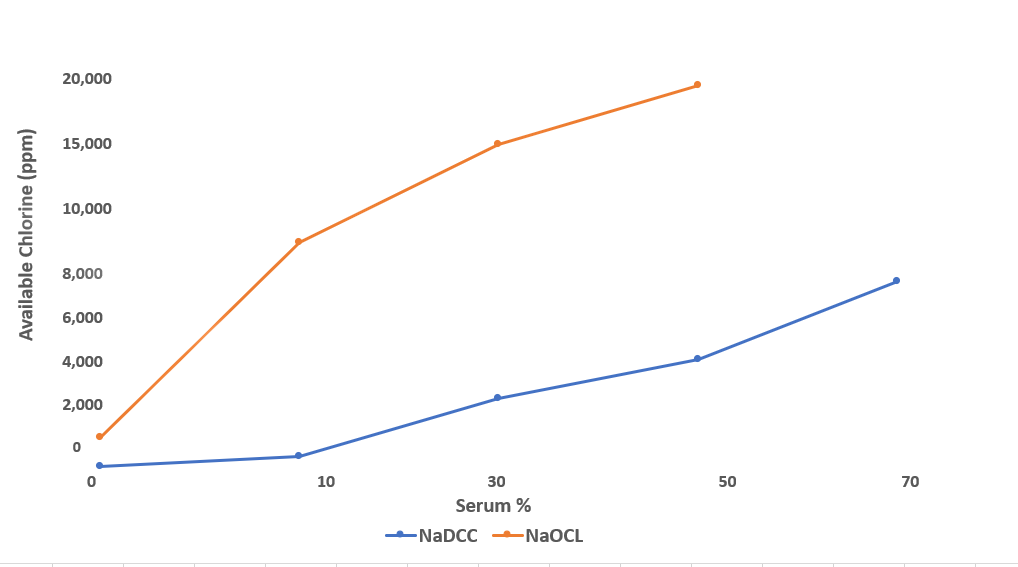
As the level of organic contamination increases, the solution strength required from hypochlorite to achieve the same killing power as NaDCC increases to over 4 times.
Comparison of the levels of available chlorine required to achieve a 99.999% kill of Pseudomonas aeruginosa in 2 minutes at 25°C in the presence of horse serum
(Coates 1988

